Previous studies had demonstrated remarkable separation of rare-earth elements by the neutral extractant Cyanex 923 using non-aqueous solvent extraction. Hence, the effects of polar organic solvents on non-aqueous solvent extraction were investigated, to enhance our understanding of the mechanisms in non-aqueous solvent extraction. The results of these studies have been published in the journal Separation and Purification Technology. The research was performed within the framework of the Horizon 2020 project NEMO.
Separation of rare-earth elements
The separation of rare-earth elements (REEs), i.e. the 15 lanthanides together with yttrium and scandium, is challenging due to close similarities in the chemical properties of adjacent elements. Conventional solvent extraction based on the selective distribution of the REEs between an aqueous phase and an immiscible organic phase is currently the technique of choice for REE separation in industry.
In non-aqueous solvent extraction (NASX), the aqueous phase is partly or completely replaced by a polar non-aqueous solvent (therefore called the more polar phase) that is largely immiscible with the less polar phase containing the extractant.
Cyanex 923 and NASX
Previous studies reported a remarkably efficient REE separation by NASX from ethylene glycol and from poly(ethylene) glycol 200 (PEG-200) using the solvating extractant Cyanex 923. To better understand these observations, our study investigated the effects of different polar molecular organic solvents on the extraction and the separation of REE chlorides by Cyanex 923. This implies that the metal ion speciation in these non-aqueous solvents must be studied, as this is essential for understanding of the NASX mechanism.
The present paper focusses specifically on ethylene glycol, as recent reports revealed significantly enhanced extraction and separation of REEs from this solvent, compared to aqueous solutions. The extraction results from a series of structural analogues, such as 1,2-propanediol and 1,3-propanediol, were compared to those of ethylene glycol. PMOSs having largely different structures and donor properties were investigated as well.
Remarkable differences were observed between the extraction behaviour from ethylene glycol, 1,2-propanediol and 1,3-propanediol. Therefore, these solvent systems were further studied to elucidate the speciation of the rare-earth elements by optical absorption and luminescence spectroscopy. Based on these studies, both contact-ion-pair formation and solvation strength are assumed to play an important role in the extraction of rare earths by Cyanex 923 from different polar organic solvents. The differences in speciation can be used as a predictive tool to fine-tune the separation of rare earths.
Full reference of paper
Brecht Dewulf, Vincent Cool, Zheng Li and Koen Binnemans, Effect of polar molecular organic solvents on non-aqueous solvent extraction of rare-earth elements, Sep. Purif. Technol., 2022, 294, 121197, https://doi.org/10.1016/j.seppur.2022.121197. (gold open access paper).
Acknowledgements
The research leading to this manuscript received funding from the European Union's EU Framework Programme for Research and Innovation Horizon 2020 under grant agreement no. 776846 (NEMO).
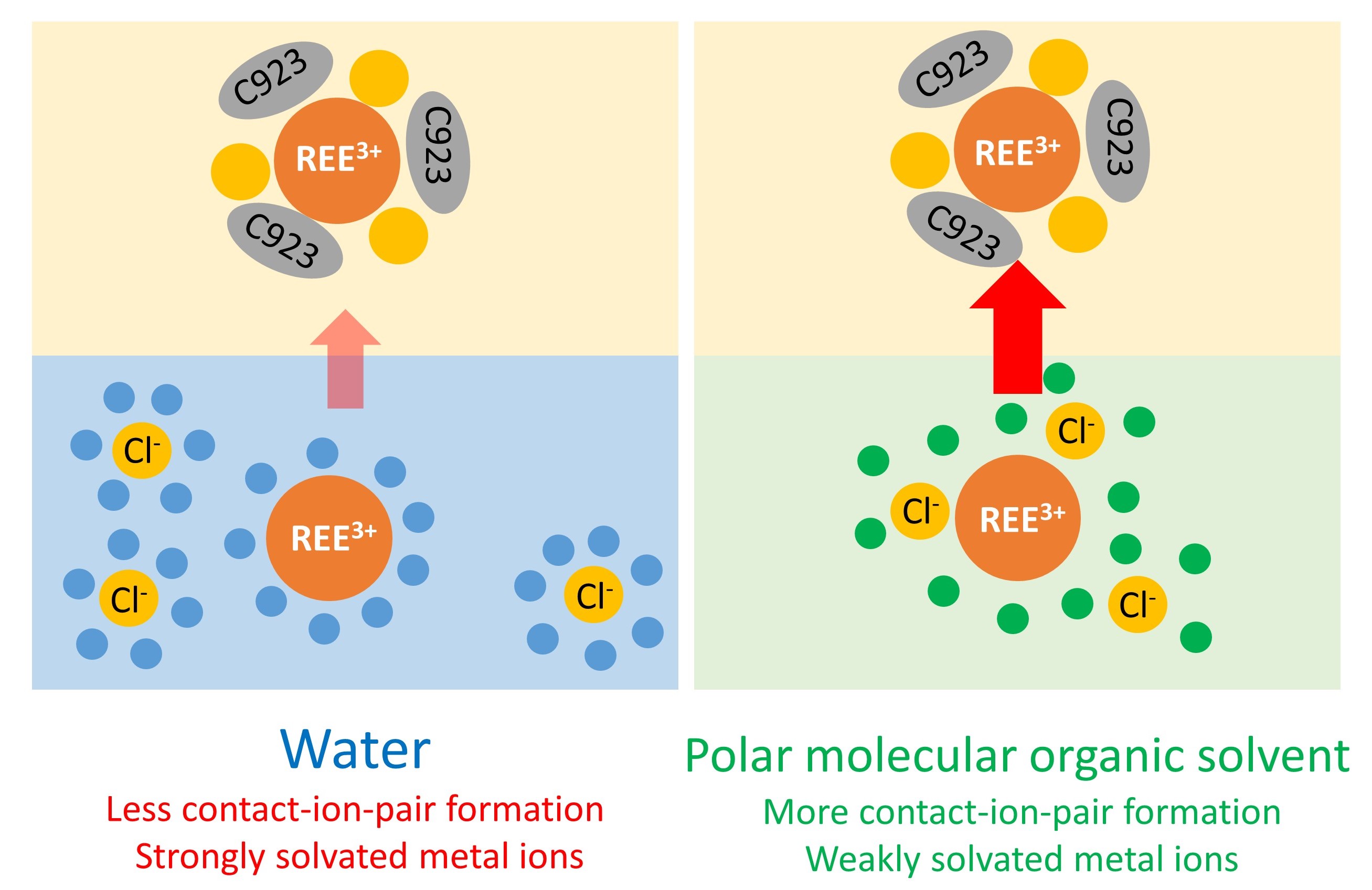
Image: Graphical abstract Brecht Dewulf et al., Sep. Purif. Technol., 2022, 294, 121197,

